Dynamics of MHC-I Patches on Cell Plasma Membranes
In collaboration with Prof. Michael Edidin, Johns Hopkins University
Our view of cell membranes as two-dimensional (2D) fluids, all the constituents of which move freely, has been changed in recent years by demonstrations of lateral heterogeneities, patches, and domains in cell surface membranes. Membrane domains have been defined mainly by measurements of the lateral diffusion of membrane proteins and lipids, although other methods have also been used. ( The measurements suggest that the size of many membrane domains is in the range of hundreds of nanometers, at or below the resolution of far-field microscopy. Near-field scanning optical microscopy (NSOM) uses the contrast techniques of far-field microscopy but achieves resolution of surface structures well beyond the diffraction limit of far-field microscopy. We therefore imaged human fibroblasts labeled with with fluorescent antibodies to membrane proteins, HLA class I (HLA-I) molecules.

Near-field images of a fixed human skin fibroblast acquired in liquid, labeled with Cy3-Fab KE-2 against HLA-I molecules. The 3D contours are from shear-force data and the color scale represents the fluorescence intensity. Topography height varies over a range of 165 nm (A) and 215 nm (B). Autocorrelation profiles and Gaussian fits for the images in A and B yield characteristic lengths of 315 nm (D), 580 nm (E).

Simulated free diffusion

Simulated obstructed diffusion (barriers)

Simulated membrane, with barriers but no vesicle traffic: no patches

Simulated membrane, with barriers AND vesicle traffic: patches are formed and maintained

Simulated membrane, with barriers and stopped vesicle traffic: patches disappear
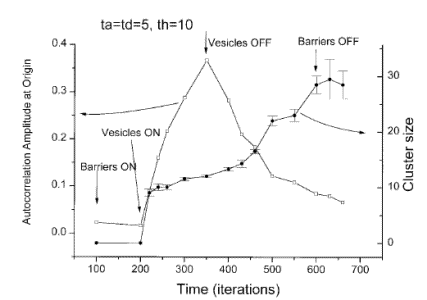
Scaling to real world parameters: a randomly organized membrane is expected to create patches of the size dictated by the barriers within 2 minutes.
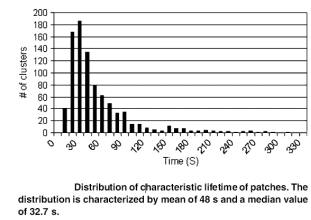
1. Patches should be dynamic in nature. They should be born, decay and disappear, then re-appear with the next vesicle delivered.
2. Patches should disappear if vesicle traffic is stopped.
3. Patches should disappear if the actin mesh is depolymerized.
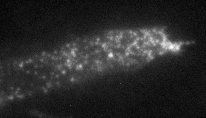
In order to check these predictions, we are using cells transfected with GFP-tagged MHC-I, and image them live with Total Internal Reflection Fluorescence Microscopy (TIRFM).

Epifluorescence
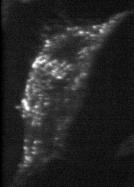
TIRFM (just the membrane)
Click image to see movie